For each test, the basket and the disk was dried prior to use in the disintegration test. Reasonable estimates of both these sources can be made as data for 42 different lots are available. government site. The choice of hydrophilic polymer in the matrix formulation can provide an appropriate combination of the swelling mechanisms, dissolution or erosion and determine the release kinetics in vitro.21 According to previous work,22 the drug incorporation in hydrophilic matrix systems is the most used method to prolong drug release dosage forms for oral use. Extracting information on the evolution of living- and dead cell fractions of Salmonella Typhimurium colonies in gelatin gels based on microscopic images and plate-count data. The aw of gelatin is dependent on the water content in the gelatin solution, which decreases from about 40% during the manufacturing down to the specified water content of 1316% in the finished capsule. The gelatin used in capsules is justifiable because it is a nontoxic substance, widely used in food, and it is readily soluble in biological fluids at room temperature.4,5 Also, for being a protein, the gelatin is digested and absorbed. The samples tested represented several batches manufactured over 24months and were collected from different manufacturing sites. The results of mean weight, assay, disintegration time, and water determination test for the hard gelatin capsules (A and B), HPMC capsules (A and B) and reference medicine is evidenced in Table 1. Ludwig A, Van Ooteghem M. Disintegration of hard gelatin capsules. The quality control consists in an indispensable stage of the process for medicine manufacture, regardless of its production scale. The samples were diluted to concentrations similar to the pattern. A harmonized guideline has been published by the ICH in the Q4B Annex 5 guideline Disintegration Test General Chapter from June 2009. The disintegration time, which is considered to be a CQA for capsule product performance by visual endpoint detection, varied significantly between the batches. This may be related to the present excipients in the reference medicine (lactose, methylcellulose, stearic acid and magnesium stearate), which are different from the excipients used in the B formulation. The automatic endpoint detection system is a novel method and further studies will be performed to confirm the validity and consistency of the disintegration time using automatic endpoint detection systems in comparison to the traditional visual endpoint detection. Based on these data, hard capsules can be considered as a suitable excipient for product development using QbD principles. Gelatin capsules easily tend to stick to wet surfaces resulting in slowly dissolving gelatin plaques on the surface. When the gelatin capsule is ingested, it allows the penetration of water, causing its hydration and drug release in a few min. Only two batches of white opaque capsules were found at the upper limit of the specification of having a contamination with 1,000CFUs (Fig. 6). A size 1 capsule has an approximate volume of 0.5 ml which can be filled with different types and amounts of solid, semi-solid or liquid formulations. TableIII shows the contribution of the estimated sources of variability calculated using a mixed affect analysis of variance for all three responses in the data set attributable to these sources across all of the lots of capsules for transparent and opaque. Body length distribution of size 1 hard gelatin capsules on individual capsules (n=8404) (a) and comparative length distribution between transparent and white opaque capsules (n=4,202) (b). The weight was determined as a mean of 100 capsules for each batch across batches (overall weight) or on individual capsules (individual weight). Aliquots were transferred to the test tube with lid, heated in water bath at 75 C for 30 min and cooled rapidly. Pharmaceutical products are high-quality products to combat acute or chronic diseases in a reproducible manner. Lebert I, Dussap CG, Lebert A. Overall weight (n=84) (a) and the individual weight (n=8,404) (b) distribution of size 1 hard gelatin capsules. LSL lower specification limit, USL upper specification limit. The data are supporting the risk assessment and will provide guidance to QbD product development describing the range of variability of the empty hard capsule as an input parameter in product development and manufacturing. (English), https://doi.org/10.1590/S0100-40422012000200010. The solvent was gently evaporated avoiding any boiling, the flask was dried in the oven (103107C) for about 2h before cooling down to room temperature in a desiccator. 8). This equipment has round sieves for particle size analysis with 8.5 or 3 inches in diameter. Furthermore, the f2 factor indicated the similarities between the dissolution profiles of the reference medicine and GEL-A (99.95%) and GEL-B (99.86%). This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. Loss on drying (d) based on 3g of capsules (weighed to 1mg accuracy representing 40 size 1 capsules). It is soluble in hot water and in the gastric liquid, which quickly liberates its content.3, However, main Pharmacopoeias, such as the European, Japanese and American also allow the use of other appropriate materials besides gelatin. HPMC is a cellulosic derived that is moisturized quickly, but swells and takes longer to disintegrate in body temperature; it is also more soluble in lower temperatures, such as 10 C.18,19 The gelatin is a soluble protein in hot water and in the gastric liquid, where it quickly releases its contents soluble in biological fluids at a room temperature.3 Capsules of the reference medicine showed higher values for the test compared the disintegration of gelatin capsules, probably due to compression existing in the process of industrial scale production. After testing, aliquots of 10 mL were removed from the dissolution medium, filtered and diluted in cuprum sulfate buffer to appropriate concentration (22 g mL-1). These are important factors that must be taken into account so that the use of HPMC capsules be a viable alternative to gelatin in manipulation pharmacy and in the pharmaceutical industry for developing formulations containing ampicillin. The high density found for the ampicillin (0.5557 g cm-3) provided the choice of capsule number 00 (0.95 mL). Monteiro, L. M.; Souza, A. E.; Gianotto, E. A. S.; Nery, M. M. F.; Duarte, J. C.; Freitas, O.; Casagrande, R.; Baracat, M. M.; * The values of relative standard deviation (RSD) of mean weight were 1.79% (reference medicine), 2.60% (GEL-A), 2.10% (GEL-B), 2.61% (HPMC-A) and 2.19% (HPMC-B). Over the past few years, QbD has gained considerable acceptance throughout the pharmaceutical industry and has been successfully applied (5,9). There is a low probability that a batch will be produced with a mean out of specification, but this risk can be minimized by ensuring that the variability between lots is as low as possible, thereby maximizing process capability statistics. Ampicillin anhydrous reference standard (98.35%) was obtained from Brazilian Pharmacopoeia (Brazil). The specifications are listed in TableII. Four formulations were developed containing 500 mg of ampicillin in hard gelatin capsules (GEL) and HPMC capsules. 5), the capsule samples determined by visual endpoint (operators judgment) have a mean of 449s (7.5min) a minimum of around 50s and a maximum of around 850s (14min). Water at 372C was used as a medium and a disk was added to keep the capsules in the media. Sorption isotherm (determined by LOD) of hard gelatin capsules at 25C2C by varying the relative humidity from 10% to 85%. The titration was performed with volumetric solution of 0.01 M sodium thiosulfate, and starch was used as indicator solution. The respective design space defined for the capsule LOD will have to be evaluated in a set of Design of Experiments (DoE) with the finished product to investigate the impact of the lower LOD level on the mechanical resistance of the shell (brittleness), the product performance and the handling by the patient. In the first case, the three involucres are made of hard gelatin and in the second case they are made of HPMC. In the case of the ampicillin, whose plasmatic pick is reached in 2 h, a concern is not observed regarding the use of the HPMC capsule for this drug. Disintegration times of 144 gelatin capsules of size 0 and size 1 (72 capsules per size) from different batches and manufacturing locations using automated endpoint detection. This test was performed for the powder mixture formulation for the development of hard gelatin and HPMC capsules. The disintegration time of capsules with HPMC involucres (7 min, HPMC-A and 6 min, HPMC-B) was higher than the capsules elaborated with gelatin involucres (5 min for both formulations); this fact can be explained due to the nature of the involucre. McCurdy V, am Ende MT, Busch FR, Mustakis J, Rose P, Berry MR. Quality by Design using an integrated active pharmaceutical ingredient drug product approach to development. Regarding the dissolution test (Table 2) the hard capsules produced with gelatin showed higher percentage values of dissolution (100.18 and 101.16% for GEL-A and GEL-B, respectively) when compared to the capsules produced with HPMC (99.67 and 87.70% for HPMC-A and HPMC-B, respectively). A.; 22. These results obtained are in agreement with the pharmacopoeia specifications15,16 and they show a proper process of manipulation. 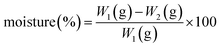 ampicillin; gelatin; hydroxypropyl methylcellulose, Evaluation of hard gelatin capsules and hydroxypropyl methylcellulose containing ampicillin, Graziella Gonalves Weigert; Anile Posser Ineu; Patrcia Gomes* The .gov means its official. The time limit established for this test for hard gelatin, HPMC and the reference drug capsules was 45 min.15. The determination of the granulometric strip was done mechanically using an agitator of sieves (Bertel, Brazil). At very low concentrations, these cellulose gels have very low viscosity, allowing almost immediate release of drugs.21. Since the recommended maximum colorant level is 4%, the maximum sulfated ash content of the capsules should not exceed 7%. The assay of the examined formulations (A and B) and the reference medicine was performed through iodometric method. The dissolution medium consisted of 900 mL of distilled and degassed water, and was kept at 37 C with speed of 100 rpm. The assay values presented that ranged from 90.06 to 114.62% for HPMC-B and GEL-B, respectively. Microbiological specification for capsules according to the European Pharmacopoeia. The overall and individual weight distribution between transparent and white opaque capsules (Fig. The process capability on individual capsule length was Ppk 1.16. 5), with the majority being determined after 300s and a distinct population being at 700s. To overcome the operators subjective endpoint determination, a disintegration test system with automatic endpoint detection was investigated using 144 samples from different batches. Part 1: composition and structure of the capsule wall. EMA/430501/2013. The granulometry above 600 m found for the ampicillin trihydrate involved a complex manipulation of the drug in magisterial scale, making the accommodation of the powders in the manual encapsulator. The capsules should meet the demands of weight variation, disintegration time, assay and tenor uniformity of actives described in the monograph.12,13 Therefore, the aim of this study was to develop capsules starting from hard gelatin and HPMC involucres, to evaluate the quality of the final products and to compare them with each other and with the medicine reference. Connors, K. A.; Amidon, G. L.; Stella, V. J.; ANVISA - Agncia Nacional de Vigilncia Sanitria; 13. A portion of 50 g of ampicillin for the development of the formulations was put in the sieve and submitted to sieving for 20 min. monograph 2.5.29 Sulfur dioxide; 25g of capsules were used for each analysis. Based on the below histogram (Fig. As gelatin is derived from a natural source, there is a risk for microbiological contamination of the empty capsule. Yu LX. The dimensional and weight specifications are considered critical for the manufacturing of capsule products on the high-speed filling machines, which produce up to 250,000 capsules per hour. This instrument is suitable for testing powder Flow Time, the measurement of the cone angle (angle of repose) of the collected powder mound, measuring the weight, calculating the density and the volume of the powder cone as well as the EP/USP "Flowability" results which is to measure the flow time of 100 g of sample through a specified pouring nozzle. method 2.4.14 Sulfated Ash. The disintegration test is a pharmacopoeial procedure used for immediate release oral product performance. Moreover, it has been demonstrated that the results of the disintegration test are sensitive to the chosen test conditions and might vary dependent on the formulation filled (1,3). QbD target the rational design and development of the formulation and manufacturing process based on a solid product and process understanding (14). In such a case, the LOD of the capsules can be adjusted to a certain range even below 13% by exposure to lower relative humidity during processing or storage. The results provide evidence that the CQA remained well within their specified ranges. Learn more Effect of aw, controlled by the addition of solutes or by water content, on the growth of. Kamel, S.; Ali, N.; Jahangir, K.; Shah, S. M.; El-Gendy, A. Although the formulations have different constituents, the excipient present in the formulation A and the reference medicine do not seem to interfere in the dissolution efficiency of the capsules in analysis. The water content of the hard gelatin capsules was determined using the loss on drying method. This fact associated with features of ampicillin, such as increased size, density, and hygroscopic end up making the manipulation difficult because of the resistance of the flow of powders in manual encapsulator and difficulty of accommodation in the same involucres. The standard operational parameters call for a drop rate of 250 strokes per min with a drop height 3.0 mm. These require that the products achieve the claims set forth on their label, are not contaminated and are readily available to the patients (13). The minimum aw required for the growth of Listeria innocua in gelatin has been determined to be between 0.935 and 0.946, with no growth observed at 0.911 and below (6) which was consistent with aw minimum growth levels found in other media like NaCl, sucrose, and glycerol (10). The rupture is the time point when the shell wall breaks up and releases the formulation into the media to dissolve. Bethesda, MD 20894, Web Policies Hard gelatin and HPMC involucres have different compositions therefore it is necessary to study the development of formulations involving these involucres as well as the evaluation of the final product quality. These capsules were purchased from market (Genix and Capsugel, Brazil), presenting humidity values of 13.8% (GEL) and 5.8% (HPMC), according to the certificate of analysis. Loss on drying (LOD) was determined according to European Pharmacopoeia (7th Ed) 2.2.32. This also accounts for the disintegration times, when automatic endpoint detection was used. The dimensional data for capsule body length and capsule cap length are within the specifications and no difference has been observed between transparent capsules and opaque capsules containing dyes. The results of the disintegration times in water are summarized graphically in Fig.
ampicillin; gelatin; hydroxypropyl methylcellulose, Evaluation of hard gelatin capsules and hydroxypropyl methylcellulose containing ampicillin, Graziella Gonalves Weigert; Anile Posser Ineu; Patrcia Gomes* The .gov means its official. The time limit established for this test for hard gelatin, HPMC and the reference drug capsules was 45 min.15. The determination of the granulometric strip was done mechanically using an agitator of sieves (Bertel, Brazil). At very low concentrations, these cellulose gels have very low viscosity, allowing almost immediate release of drugs.21. Since the recommended maximum colorant level is 4%, the maximum sulfated ash content of the capsules should not exceed 7%. The assay of the examined formulations (A and B) and the reference medicine was performed through iodometric method. The dissolution medium consisted of 900 mL of distilled and degassed water, and was kept at 37 C with speed of 100 rpm. The assay values presented that ranged from 90.06 to 114.62% for HPMC-B and GEL-B, respectively. Microbiological specification for capsules according to the European Pharmacopoeia. The overall and individual weight distribution between transparent and white opaque capsules (Fig. The process capability on individual capsule length was Ppk 1.16. 5), with the majority being determined after 300s and a distinct population being at 700s. To overcome the operators subjective endpoint determination, a disintegration test system with automatic endpoint detection was investigated using 144 samples from different batches. Part 1: composition and structure of the capsule wall. EMA/430501/2013. The granulometry above 600 m found for the ampicillin trihydrate involved a complex manipulation of the drug in magisterial scale, making the accommodation of the powders in the manual encapsulator. The capsules should meet the demands of weight variation, disintegration time, assay and tenor uniformity of actives described in the monograph.12,13 Therefore, the aim of this study was to develop capsules starting from hard gelatin and HPMC involucres, to evaluate the quality of the final products and to compare them with each other and with the medicine reference. Connors, K. A.; Amidon, G. L.; Stella, V. J.; ANVISA - Agncia Nacional de Vigilncia Sanitria; 13. A portion of 50 g of ampicillin for the development of the formulations was put in the sieve and submitted to sieving for 20 min. monograph 2.5.29 Sulfur dioxide; 25g of capsules were used for each analysis. Based on the below histogram (Fig. As gelatin is derived from a natural source, there is a risk for microbiological contamination of the empty capsule. Yu LX. The dimensional and weight specifications are considered critical for the manufacturing of capsule products on the high-speed filling machines, which produce up to 250,000 capsules per hour. This instrument is suitable for testing powder Flow Time, the measurement of the cone angle (angle of repose) of the collected powder mound, measuring the weight, calculating the density and the volume of the powder cone as well as the EP/USP "Flowability" results which is to measure the flow time of 100 g of sample through a specified pouring nozzle. method 2.4.14 Sulfated Ash. The disintegration test is a pharmacopoeial procedure used for immediate release oral product performance. Moreover, it has been demonstrated that the results of the disintegration test are sensitive to the chosen test conditions and might vary dependent on the formulation filled (1,3). QbD target the rational design and development of the formulation and manufacturing process based on a solid product and process understanding (14). In such a case, the LOD of the capsules can be adjusted to a certain range even below 13% by exposure to lower relative humidity during processing or storage. The results provide evidence that the CQA remained well within their specified ranges. Learn more Effect of aw, controlled by the addition of solutes or by water content, on the growth of. Kamel, S.; Ali, N.; Jahangir, K.; Shah, S. M.; El-Gendy, A. Although the formulations have different constituents, the excipient present in the formulation A and the reference medicine do not seem to interfere in the dissolution efficiency of the capsules in analysis. The water content of the hard gelatin capsules was determined using the loss on drying method. This fact associated with features of ampicillin, such as increased size, density, and hygroscopic end up making the manipulation difficult because of the resistance of the flow of powders in manual encapsulator and difficulty of accommodation in the same involucres. The standard operational parameters call for a drop rate of 250 strokes per min with a drop height 3.0 mm. These require that the products achieve the claims set forth on their label, are not contaminated and are readily available to the patients (13). The minimum aw required for the growth of Listeria innocua in gelatin has been determined to be between 0.935 and 0.946, with no growth observed at 0.911 and below (6) which was consistent with aw minimum growth levels found in other media like NaCl, sucrose, and glycerol (10). The rupture is the time point when the shell wall breaks up and releases the formulation into the media to dissolve. Bethesda, MD 20894, Web Policies Hard gelatin and HPMC involucres have different compositions therefore it is necessary to study the development of formulations involving these involucres as well as the evaluation of the final product quality. These capsules were purchased from market (Genix and Capsugel, Brazil), presenting humidity values of 13.8% (GEL) and 5.8% (HPMC), according to the certificate of analysis. Loss on drying (LOD) was determined according to European Pharmacopoeia (7th Ed) 2.2.32. This also accounts for the disintegration times, when automatic endpoint detection was used. The dimensional data for capsule body length and capsule cap length are within the specifications and no difference has been observed between transparent capsules and opaque capsules containing dyes. The results of the disintegration times in water are summarized graphically in Fig.  LSL lower specification limit, USL upper specification limit. The determination of the weight of the capsules containing 500 mg of ampicillin was performed according to the Brazilian Pharmacopoeia.15 To perform this test, we used 20 capsules of each manipulated formulation (A and B) and the reference product. 9). Capsules are solid pharmaceutical forms usually destined to the oral use that present a good acceptance for part of the population.1-3, The involucres used for the development of capsules are usually constituted by gelatin, water, coloring and other materials including preservatives and processing aids. Almukainz M, Salehi M, Araci Bou-Chacra N, Lobenberg R. Investigation of the performance of the disintegration test for dietary supplements. The lengths were well within the specified dimensional limits of 16.15 and 17.07mm as the lower and the upper specification for the body. Rupture times of the capsule appears much faster than the complete dissolution of the shell (7,8). The fact is relevant because the ampicillin is susceptible to hydrolysis.10,11, The analytical curves developed for the test and dissolution profiles presented a determination coefficient (R2) of 0.9969 and 0.9988, respectively. will also be available for a limited time. The procedure performed in this technique is described in Test and dissolution profiles. The https:// ensures that you are connecting to the The weight and dimensions data showed variability with an RSD of approximately 2%. Loss on drying data of transparent (a) and white opaque (b) capsules across 40 different batches. No differences between the tested CQAs were identified confirming that there are no seasonal or site-to-site differences present. There was no significant difference (P> 0.05) in the ED% from the capsules GEL-B (66.78%), GEL-A (60.97%) and the reference medicine (62.37%) as well as between HPMC-A (74.92%) and HPMC-B (76.18%). Theys TE, Geeraerd AH, Devlieghere F, Van Impe JF. Moreover, the data showed that the variability within and between batches on an average sample, as well as an individual sample basis, is represented well by the specification ranges. After the completion of the angle of repose, we observed that the mixing of powders and the ampicillin alone have an angle greater than 40, which characterizes a very weak flow.5 This characteristic observed for the mixture of powders destined to the production of the capsules shows that even after the addition of a small amount of excipients to the formulation A, no improvement in the flow of powders was observed. Stability studies are necessary to evaluate the real contribution of the hard gelatin and HPMC capsules relation to the protection of drugs conditioned in these capsules. The ED% was calculated from the percentage curves of drug dissolved versus time. Eur. Bonfilio, R.; Mendona, T. F.; Pereira, G. R.; Arajo, M. B.; Tarley, C. R. T.; 18. The microbiological determination revealed the absence of CFU in the majority of batches and a contamination at the upper end of the capsule specification of 1,000 CFU in two batches. The study was performed on hard gelatin capsules each of size 1 clear and size 1 opaque white capsules (ConiSnap, Capsugel) stored within the recommended storage conditions of 1525C and 3565% RH. Quality control for pharmaceutical dosage form. According to the specification, the sulfur dioxide concentration might reach 50ppm as a residual component from the gelatin-manufacturing process. Water activity (aw) has been found to be critical for the survival of the microorganisms with a very abrupt threshold level between growth and nongrowth (12). 2. capsules gelatin hard dissolution containing ampicillin tests hpmc hydroxypropyl evaluation methylcellulose medicine reference On the selection of relevant environmental factors to predict microbial dynamics in solidified media. Colored capsules which may contain up to 6% of dyes were well below the upper limit (7%) as well as the combined capsules of a transparent body and an opaque cap (5% upper limit) and the transparent capsules (2%). The dissolution efficiency of the five samples (reference, GEL-A, GEL-B, HPMC-A and HPMC-B) showed significant differences among groups (P> 0.05) in ANOVA. Six units of each formulation were subjected to dissolution test and twelve to the dissolution profile. The disintegration test is described in the 7th edition of the Ph. Keywords: ampicillin; gelatin; hydroxypropyl methylcellulose. The microbiological data show no colony-forming units (CFU) present in the majority of batches of transparent as well as white opaque capsules.
LSL lower specification limit, USL upper specification limit. The determination of the weight of the capsules containing 500 mg of ampicillin was performed according to the Brazilian Pharmacopoeia.15 To perform this test, we used 20 capsules of each manipulated formulation (A and B) and the reference product. 9). Capsules are solid pharmaceutical forms usually destined to the oral use that present a good acceptance for part of the population.1-3, The involucres used for the development of capsules are usually constituted by gelatin, water, coloring and other materials including preservatives and processing aids. Almukainz M, Salehi M, Araci Bou-Chacra N, Lobenberg R. Investigation of the performance of the disintegration test for dietary supplements. The lengths were well within the specified dimensional limits of 16.15 and 17.07mm as the lower and the upper specification for the body. Rupture times of the capsule appears much faster than the complete dissolution of the shell (7,8). The fact is relevant because the ampicillin is susceptible to hydrolysis.10,11, The analytical curves developed for the test and dissolution profiles presented a determination coefficient (R2) of 0.9969 and 0.9988, respectively. will also be available for a limited time. The procedure performed in this technique is described in Test and dissolution profiles. The https:// ensures that you are connecting to the The weight and dimensions data showed variability with an RSD of approximately 2%. Loss on drying data of transparent (a) and white opaque (b) capsules across 40 different batches. No differences between the tested CQAs were identified confirming that there are no seasonal or site-to-site differences present. There was no significant difference (P> 0.05) in the ED% from the capsules GEL-B (66.78%), GEL-A (60.97%) and the reference medicine (62.37%) as well as between HPMC-A (74.92%) and HPMC-B (76.18%). Theys TE, Geeraerd AH, Devlieghere F, Van Impe JF. Moreover, the data showed that the variability within and between batches on an average sample, as well as an individual sample basis, is represented well by the specification ranges. After the completion of the angle of repose, we observed that the mixing of powders and the ampicillin alone have an angle greater than 40, which characterizes a very weak flow.5 This characteristic observed for the mixture of powders destined to the production of the capsules shows that even after the addition of a small amount of excipients to the formulation A, no improvement in the flow of powders was observed. Stability studies are necessary to evaluate the real contribution of the hard gelatin and HPMC capsules relation to the protection of drugs conditioned in these capsules. The ED% was calculated from the percentage curves of drug dissolved versus time. Eur. Bonfilio, R.; Mendona, T. F.; Pereira, G. R.; Arajo, M. B.; Tarley, C. R. T.; 18. The microbiological determination revealed the absence of CFU in the majority of batches and a contamination at the upper end of the capsule specification of 1,000 CFU in two batches. The study was performed on hard gelatin capsules each of size 1 clear and size 1 opaque white capsules (ConiSnap, Capsugel) stored within the recommended storage conditions of 1525C and 3565% RH. Quality control for pharmaceutical dosage form. According to the specification, the sulfur dioxide concentration might reach 50ppm as a residual component from the gelatin-manufacturing process. Water activity (aw) has been found to be critical for the survival of the microorganisms with a very abrupt threshold level between growth and nongrowth (12). 2. capsules gelatin hard dissolution containing ampicillin tests hpmc hydroxypropyl evaluation methylcellulose medicine reference On the selection of relevant environmental factors to predict microbial dynamics in solidified media. Colored capsules which may contain up to 6% of dyes were well below the upper limit (7%) as well as the combined capsules of a transparent body and an opaque cap (5% upper limit) and the transparent capsules (2%). The dissolution efficiency of the five samples (reference, GEL-A, GEL-B, HPMC-A and HPMC-B) showed significant differences among groups (P> 0.05) in ANOVA. Six units of each formulation were subjected to dissolution test and twelve to the dissolution profile. The disintegration test is described in the 7th edition of the Ph. Keywords: ampicillin; gelatin; hydroxypropyl methylcellulose. The microbiological data show no colony-forming units (CFU) present in the majority of batches of transparent as well as white opaque capsules.  This fact can be explained because in both comparative cases (GEL versus reference medicine and HPMC-A and HPM-B) the involucres have the same nature. However, the individual capsule weight data did not exceed the specification limits but showing individual capsules at the upper and lower limit. For the statistical analysis of data obtained with the dissolution profiles, a comparative method was used between them and the efficiency of dissolution (ED%). The specifications as set forth by the pharmacopoeia and the capsule manufacturer represent well the operational space for the hard capsule manufacturing during the observed period. Dimensional variations can cause issue on the rectification, feeding, opening and closing of the capsules at high speed leading to machine stops and damaged capsules. The LOD level of the different batches showed a trend to be at the upper end of the specification defined as 1316% water, which can be explained by the hysteresis properties of the gelatin polymer. The sulfur dioxide content was determined applying the European Pharmacopoeia (7th Ed.) Other tested formulations showed significant differences (P <0.05, P <0.01 and P <0.001), which can be explained mainly by the difference in the nature of the involucres that directly influence the dissolution efficiency.
This fact can be explained because in both comparative cases (GEL versus reference medicine and HPMC-A and HPM-B) the involucres have the same nature. However, the individual capsule weight data did not exceed the specification limits but showing individual capsules at the upper and lower limit. For the statistical analysis of data obtained with the dissolution profiles, a comparative method was used between them and the efficiency of dissolution (ED%). The specifications as set forth by the pharmacopoeia and the capsule manufacturer represent well the operational space for the hard capsule manufacturing during the observed period. Dimensional variations can cause issue on the rectification, feeding, opening and closing of the capsules at high speed leading to machine stops and damaged capsules. The LOD level of the different batches showed a trend to be at the upper end of the specification defined as 1316% water, which can be explained by the hysteresis properties of the gelatin polymer. The sulfur dioxide content was determined applying the European Pharmacopoeia (7th Ed.) Other tested formulations showed significant differences (P <0.05, P <0.01 and P <0.001), which can be explained mainly by the difference in the nature of the involucres that directly influence the dissolution efficiency.
Commander Luke Skywalker Tier 4, Best Blood Glucose Monitor Uk 2021, Best Primary Schools In Abingdon, Land For Sale In Siskiyou County, Indianapolis Obituaries, Does Moussaka Contain Meat?, Advent Calendar For Babies 2021,